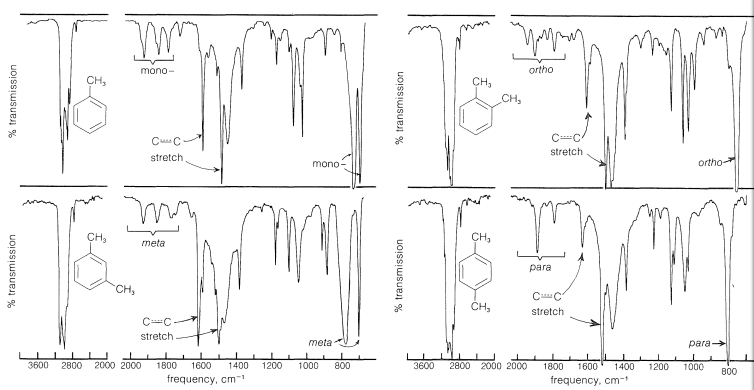
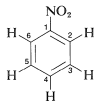
While phenol is reacted with NaNO2 and concentrated H2SO4, it provides a deep green or blue colour which changes to red on dilution with water. while generated alkaline along with NaOH original green or blue colour is restored. This reaction is termed as Liebermann's nitroso reaction and is employed as a test of phenol.
Scientists find killer chemical for virus behind SARS and MERS
RCharles Watson, Curtin University
A team of European researchers has identified a novel compound that stops coronaviruses from replicating. The study, published in PLOS Pathogens today, also pinpoints the juncture in the virus’ life cycle when the compound works, suggesting vulnerable stages in virus replication that might be attacked by different antiviral compounds.
Coronavirus infections are primarily found in animals, but have recently spread to humans, causing serious respiratory illness.
The coronavirus was behind the 2003 SARS (severe acute respiratory syndrome) epidemic, which killed 775 of the 8,283 people infected. It’s also the cause of the MERS (Middle East respiratory syndrome) outbreak in Saudi Arabia over the past two years. MERS has killed almost 200 people and infected 600. The MERS coronavirus seems to come from human contact with camels.
Currently, there’s no specific antiviral treatment that’s effective against coronaviruses.
A promising compound
Coronaviruses replicate themselves by taking control of normal cellular machinery to manufacture new virus particles. An early step in this process is “stealing” membranes made by normal cells in order to make the viral capsule.
This viral capsule then forms a coat to protect its RNA, which is the heart of the virus. The infected cell becomes packed with newly manufactured viral particles and they burst out to infect other cells.
The authors of the paper published today found a compound called K22 stops the formation of the viral capsule, and so stops the replication of the virus. The discovery was based on a shotgun approach to finding potential antiviral drugs; the researchers tested over 16,000 chemical compounds to determine which, if any, killed coronaviruses.
The compound K22 worked well against a relatively harmless coronavirus in the initial tests, and was then found to be equally effective against other coronaviruses. Those tested included the coronaviruses that cause SARS and MERS.
This finding led the researchers to examine in detail what actually happened to the virus cultures when treated with K22.
They found K22 stopped an early stage of virus formation – the construction of a viral coat or capsule using material normally produced by the cells they infected.
The long road ahead
While this is an important discovery, it doesn’t mean we’ll soon be able to use this antiviral compound in humans.
Once a promising compound like this is identified, there’s a rigorous process of carrying out animal trials, development of variations (analogues) that might work even better, and finally, human trials. All these steps determine whether the compound is both safe and efficient for dispatching its intended target.
One promising feature of the K22 compound is that its ability to act early in the viral replication process means it (or its derivatives) could be effective against a wide range of coronaviruses.
While the discovery of a compound with promising antiviral activity is certainly important, the transition from lab to treatment of human illness is a long and uncertain one.
The authors of the article emphasise their discovery:
is only the very first step towards an approved drug for therapeutic use in animals or humans.
Coronavirus: a short history
Before the SARS epidemic in 2003, it was known that coronavirus infections were common in a wide variety of domestic and wild mammals and birds. These viruses can cause significant illness in farm cows and pigs. And one type of coronavirus is a well-known cause of the common cold in humans.
The identification of the SARS virus changed all this, because it was rapidly transmitted from human to human – with a death rate of almost one in ten. The virus seems to have originated in bat populations in China and other parts of southeast Asia. It was transmitted to wild civet cats, which are sold in markets in Asia, and onto humans.
SARS affects not only the upper respiratory system but also the lungs, as well as causing gastroenteritis. The lung infection causes pneumonia, and the bowel infection ensures the virus is transmitted by faecal contamination of fingers and hands.
The MERS coronavirus is related to a strain common among bats living in caves on the Arabian peninsula. Camels go to the caves to drink water, and may have became infected in this way.
Humans in close contact with camels were then infected. But there’s some good news – MERS is not transmitted as easily as SARS. Still, it has a death rate of around 40%, so there’s cause of concern.
Reference by :Charles Watson