Hii dear friends
I am going to share some idea about Friedel-Crafts reaction how its work i hope so its very help for you people.
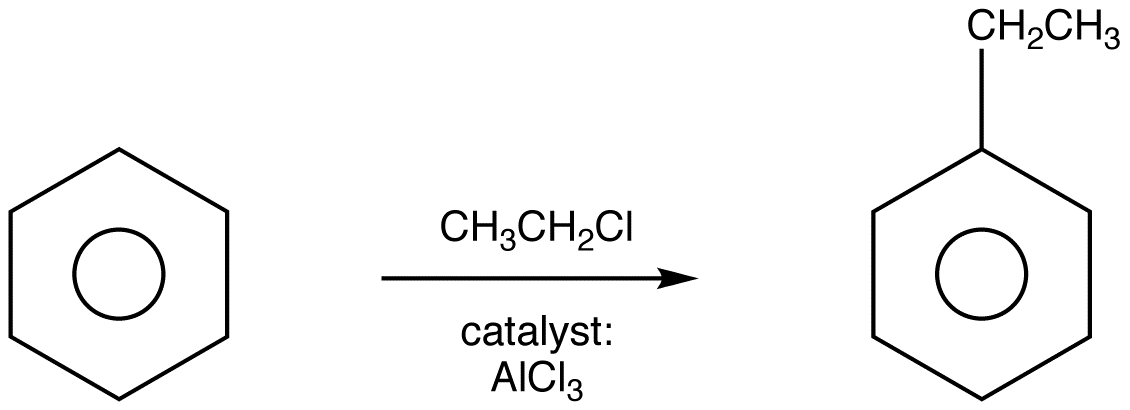
Friedel-Crafts Alkylation
Friedel-Crafts Alkylation was first discovered by French scientist Charles Friedel and his partner, American scientist James Crafts, in 1877. This reaction allowed for the formation of alkyl benzenes from alkyl halides, but was plagued with unwanted supplemental activity that reduced its effectivity
The mechanism takes place as follows
Step 1:
.png?revision=1)
Step one creates a carbocation that acts as the electrophile in the reaction. This steps activates the haloalkane. Secondary and tertiary halides only form the free carbocation in this step
Steps 2 and 3
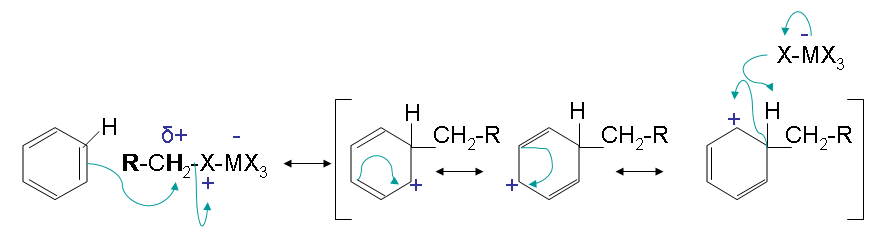
Step 2 has an electrophilic attack on the benzene resulting in multiple resonance forms. The halogen reacts with the intermediate and picks up the hydrogen to eliminate the positively charge
Final Products
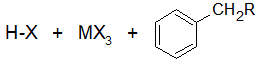
The reactivity of haloalkanes increases as you move up the periodic table and increase polarity. This means that an RF haloalkane is most reactive followed by RCl then RBr and finally RI. This means that the Lewis acids used as catalysts in Friedel-Crafts Alkylation reactions tend have similar halogen combinations such as BF3, SbCl5, AlCl3, SbCl5, and AlBr3, all of which are commonly used in these reactions
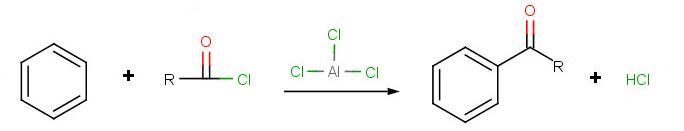
Friedel-Crafts Acylation
The goal of the reaction is the following:
.jpg?revision=1)
The very first step involves the formation of the acylium ion which will later react with benzene
The second step involves the attack of the acylium ion on benzene as a new electrophile to form one complex:
.jpg?revision=1)
The third step involves the departure of the proton in order for aromaticity to return to benzene:
During the third step, AlCl4 returns to remove a proton from the benzene ring, which enables the ring to return to aromaticity. In doing so, the original AlCl3 is regenerated for use again, along with HCl. Most importantly, we have the first part of the final product of the reaction, which is a ketone. Thie first part of the product is the complex with aluminum chloride as shown:
.jpg?revision=1)
The fiynal step involves the addition of water to liberate the final product as the acylbenzene:
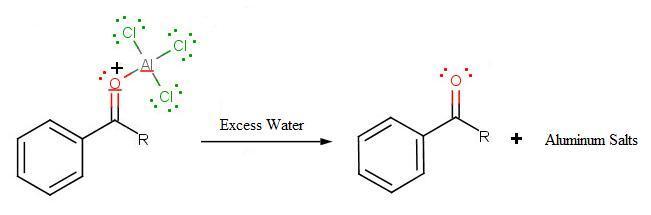.jpg?revision=1)
Because the acylium ion (as was shown in step one) is stabilized by resonance, no rearrangement occurs (Limitation 1). Also, because of of the deactivation of the product, it is no longer susceptible to electrophilic attack and hence, is no longer susceptible to electrophilic attack and hence, no longer goes into further reactions (Limitation 3). However, as not all is perfect, Limitation 2 still prevails where Friedel-Crafts Acylation fails with strong deactivating rings
.jpg?revision=1)



Sir aaj jake samja friedle craft acylation tnx sir
ReplyDelete